Feb 5, 2026
Designing next-generation drugs increasingly depends on our ability to model complex molecular systems with extreme precision.
We are excited to announce a new milestone in molecular quantum computing: a high-accuracy hybrid quantum–classical algorithm for modeling relativistic spin interactions in molecules, a long-standing challenge in computational chemistry.
This advance represents a crucial step toward more predictive simulations for drug discovery and materials design.
Led by Dr. Emil Żak, this work demonstrates how combining advanced classical computation techniques with fault-tolerant quantum algorithms can push beyond the limits of either approach alone. By unifying classical and quantum computing within a single workflow, we show a practical path toward tackling chemically and pharmaceutically relevant problems that have remained computationally inaccessible.
This hybrid approach opens the door to more accurate modeling of drug candidates where existing methods fall short, helping reduce uncertainty earlier in the discovery pipeline.
Why Relativistic Spin Effects Matter
Understanding how an electron’s spin influences chemical behavior is essential whenever heavy atoms or spin-sensitive transitions are involved. These subtle relativistic spin effects drive phenomena ranging from how cancer treatments generate cell-killing oxygen to how catalysts convert CO₂ to fuel. In photodynamic therapy (a light-activated cancer treatment) or spin-dependent catalysis, the outcome of a reaction can hinge on these quantum spin interactions.
However, capturing spin effects accurately in simulations has been notoriously difficult. Including spin-orbit and spin-spin interactions causes an additional computational overhead on top of the explosive exponential growth in complexity with increasing system size. Because the problem’s size blows up exponentially, and even the world’s most powerful supercomputers struggle beyond the smallest molecules. In practice, chemists have had to rely on simplifying assumptions or omitting spin terms altogether, which means sacrificing accuracy in exactly those cases where spin matters most. The result is a major blind spot in molecular design: many phenomena involving spin (so-called “spin-forbidden” processes) remain inaccessible to classical computation under realistic conditions. This is exactly where quantum computing offers a way forward.
A New Hybrid Quantum-Classical Approach
Our team has developed a fault-tolerant quantum algorithm tailored to the Pauli-Breit Hamiltonian - the comprehensive physics equation that includes all those relativistic spin interactions. In simple terms, this algorithm allows a quantum computer to natively simulate a molecule’s electrons with spin-orbit coupling and spin-spin effects fully accounted for. Instead of resorting to approximations, we encode the complete relativistic molecular Hamiltonian into quantum circuits efficiently (using a technique called block-encoding). We also designed new circuit tricks to handle spin elegantly, such as spin-controlled Pauli-SWAP networks that incorporate spin logic with only minimal quantum gate overhead.
Our approach introduces several key innovations:
Explicit Quantum Encoding of Spin Interactions - We embed the full Pauli–Breit relativistic Hamiltonian into a quantum circuit, capturing both one-electron and two-electron spin-orbit coupling exactly (no more averaging or guessing). This means the quantum simulation treats spin effects with the same first-principles accuracy as other forces in the molecule, instead of using rough approximations.
Spin-Optimized Quantum Circuits - We employ spin-controlled Pauli-SWAP networks - a novel circuit design that separates spin control from orbital logic. This allows a unified treatment of spin mixing with only a modest extra cost relative to a spin-free simulation. In fact, including explicit spin degrees of freedom does not worsen the algorithm’s scaling; we proved the asymptotic complexity remains the same, and even reduced certain costs by about a factor of two compared to a naive approach. In short, our quantum algorithm can incorporate spin without blowing up the resource requirements.
Integrated Classical Workflow (AI + Quantum) - Uniquely, we’ve connected our quantum algorithm to classical computation in a seamless workflow. After the quantum step computes high-fidelity spin-dependent properties for a set of molecules, those results are fed into a classical graph neural network (GNN) model. The AI model learns from a few hundred quantum-calculated examples and can then predict the same properties for thousands of new molecular candidates almost instantly. This hybrid pipeline leverages quantum accuracy for a small training set, and classical machine learning for massive scale-up - enabling high-throughput screening of chemical libraries that would be impractical to simulate one by one.
Crucially, these innovations mean we can now calculate from first principles the very quantities that were once out of reach. Our quantum algorithm can output key molecular properties governed by spin - for example, the precise energy levels of a molecule’s singlet and triplet states, the strength of spin-orbit coupling between those states, and even the expected intersystem crossing rate (how fast a molecule transitions between spin states). Such detailed spin-resolved data are the “holy grail” that classical methods struggle to obtain. By marrying this quantum capability with classical AI for scale, we unlock a practical route to design and discover molecules with optimized spin-dependent behavior.
Real-World Impact: Cancer Therapy and Clean Energy
Photodynamic therapy (PDT) - an emerging cancer treatment - is a perfect example of where this quantum-classical approach can make a difference. PDT uses special molecules called photosensitizers that, when activated by a laser, produce reactive oxygen species (ROS) to kill tumor cells. It’s a highly targeted therapy: only cells in the illuminated tumor region are destroyed, sparing healthy tissue. However, today’s photosensitizer drugs have limitations. Many have insufficient triplet yields (they don’t convert to the ROS-generating triplet state efficiently), degrade under light, or require light frequencies that can’t penetrate deeply into tissue. These issues - poor ROS generation, photostability, shallow light penetration - all trace back to the molecule’s fundamental excited-state properties, especially the efficiency of intersystem crossing (the spin-flip process that creates the triplet state) and the energy levels involved. In other words, improving PDT drugs is fundamentally a physics and chemistry problem: we need to engineer molecules with the right spin-dependent photophysics.
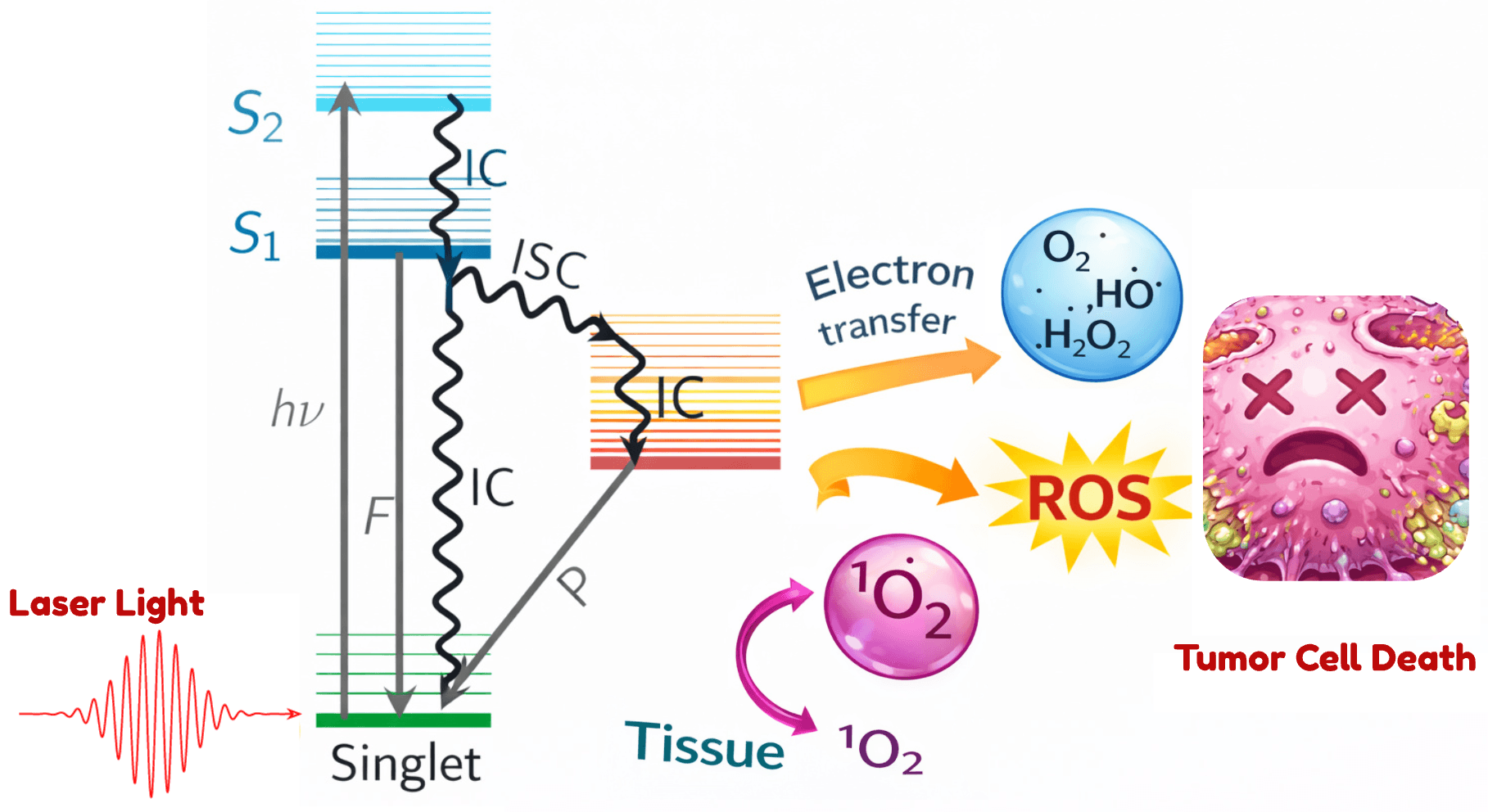
Figure: Schematic of intersystem crossing and ROS generation in photodynamic therapy. A photosensitizer molecule (left) is excited by a laser from its ground singlet state to excited singlet states (S₁, S₂). Throughintersystem crossing(ISC, wiggly arrow), the molecule transitions into a triplet state, which can then transfer energy to oxygen in tissue to producereactive oxygen species(ROS, e.g. singlet oxygen ¹O₂). These ROS go on to kill the tumor cell (right).
Our hybrid quantum-classical approach directly targets these spin-dependent factors. Using the quantum algorithm, we can calculate a candidate drug’s photophysical properties from first principles - for example, the exact singlet and triplet energy levels, the spin-orbit coupling strength between them, and the predicted rate of intersystem crossing (which dictates how much ROS gets produced). Armed with these insights, we can then rationally design new photosensitizer molecules rather than relying on trial and error. For instance, if a particular molecular structure shows a higher ISC rate and a triplet energy aligned with efficient ROS generation, that structure can be prioritized for development.
Thanks to the integrated GNN model, we don’t have to run the quantum algorithm on every single candidate (which would be slow); instead, the AI predicts which chemical modifications or new structures will yield the desired spin properties, after learning from a smaller quantum-computed dataset. This drastically accelerates the search. In practical terms, this means we could screen thousands of potential PDT compounds in silico, pinpointing the most promising ones that combine strong spin-orbit coupling (for efficient ISC), photochemical stability, and tissue-penetrating absorption wavelengths. Such a capability is unprecedented - currently, no classical chemistry software can fully account for these relativistic spin effects in drug molecules. By bringing quantum computing into the loop, we open a path to discover cancer-fighting molecules that might have been missed by conventional methods.
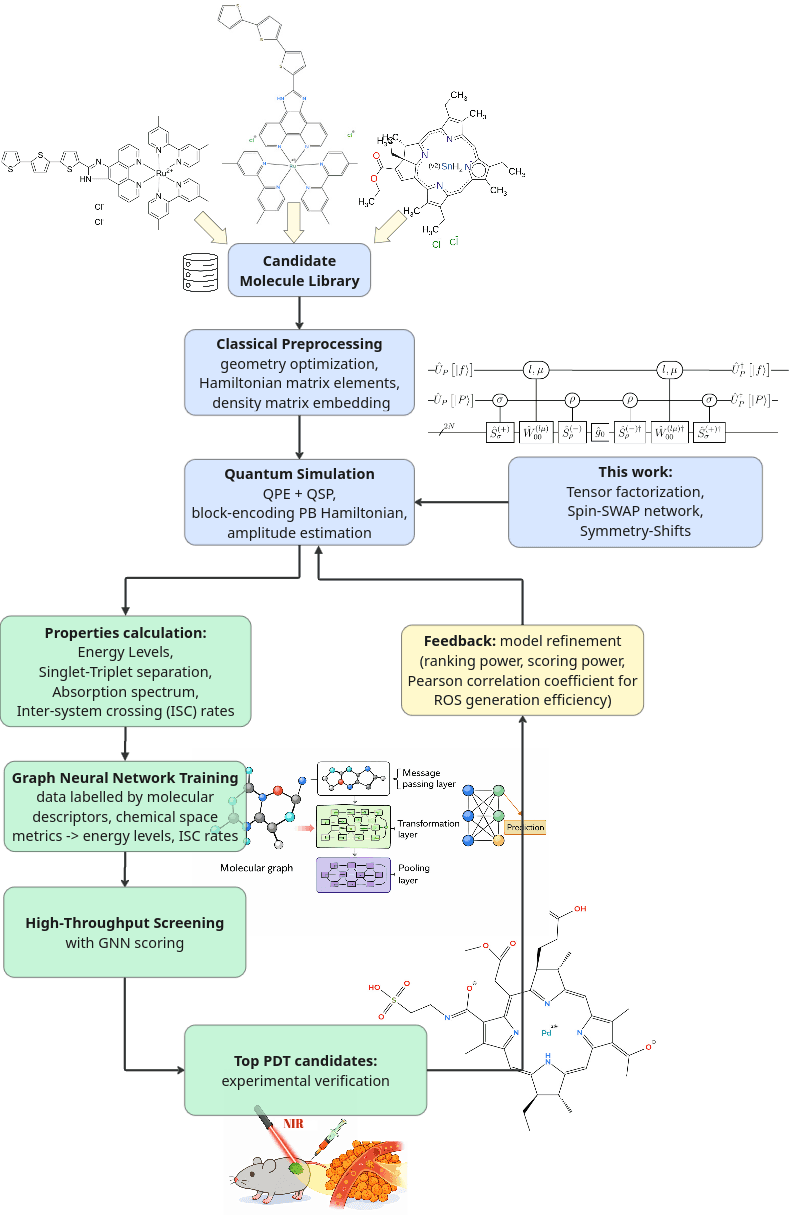
Figure: High-level workflow of the hybrid quantum-classical pipeline for photodynamic therapy drug design. A fault-tolerant quantum algorithm computes key photophysical properties (singlet/triplet energies, spin-orbit couplings and intersystem crossing rates) for a selection of candidate molecules. These quantum-derived results then train a graph neural network model, which can rapidly predict properties for large libraries of new molecules. The GNN-guided screening identifies top candidates with desired absorption and ROS-generating characteristics. Subsequent experimental feedback helps establish key metrics for quantum model refinement.
Beyond cancer therapy, the same hybrid approach extends to other domains where electrons’ spins play a crucial role. In artificial photosynthesis and catalysis, for example, chemists often introduce heavy atoms (like ruthenium or iridium) into catalysts to enhance spin-orbit interactions and improve reaction efficiencies. Our algorithm can evaluate such spin-mediated catalysis mechanisms with full accuracy, helping design better catalysts for clean energy production (such as more efficient CO₂-to-fuel conversion catalysts). Likewise, any process involving spin-forbidden transitions - from certain types of high-resolution spectroscopy to magnetic material design - could benefit from the ability to simulate spin dynamics exactly. In short, by capturing relativistic spin effects, we unlock new possibilities to innovate in both healthcare and sustainable technology, bridging a critical gap that classical computation alone could not cross.
The Road Ahead
While our hybrid quantum-classical solution is a significant breakthrough, it’s important to note that executing it in practice will require advances in quantum hardware. The algorithm is designed for fault-tolerant quantum computers - the error-corrected, next-generation devices that are still under development. Current quantum processors are too noise-prone and limited in qubit count to run these large-scale simulations. Moreover, the full workflow (quantum phase estimation, loading molecular data, error correction, etc.) involves a substantial computational overhead. We have worked hard to minimize the extra cost of handling spin (our approach makes the “spin-included” problem about as hard as the spin-free one, with only a small constant-factor increase), but the absolute requirements are still high.
The good news is that this research provides a clear target for quantum hardware development and software optimization. As quantum technology improves, our algorithm can scale to increasingly complex molecules, and we are actively exploring ways to further reduce the resource demands in the meantime. Our ongoing work aims to streamline data encoding, optimize circuits, and integrate our method into user-friendly tools. We are also reaching out to collaborators in both industry and academia to begin testing parts of this workflow on today’s best quantum emulators and early hardware, as well as to incorporate experimental feedback on candidate molecules.
Every step we take now is building the foundation for a future where quantum computing and classical computing work hand-in-hand to solve real-world problems. This hybrid approach exemplifies how quantum advantage can emerge not in isolation, but in concert with classical techniques - delivering practical solutions faster and more efficiently. We believe that by working together across disciplines, we can harness this quantum-classical synergy to address pressing challenges like cancer treatment and clean energy. It’s an invitation to the broader community: join us in translating these cutting-edge quantum algorithms into tangible benefits for society. Together, we can make quantum computations for a better world.
(For those interested in technical details, we invite you to read the full research preprint available on arXiv.)

